Heat Definition Unit . the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. temperature measures the average kinetic energy of the molecules. heat and temperature are two different but closely related concepts. Note that they have different units: Another unit is the calorie (cal), with 1. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. The kilocalorie ( 1 kcal = 1000 cal) is the. units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. the standard unit of heat in the international system of units (si) is the joule (j). When the temperature rises, the molecules become.
from studylib.net
temperature measures the average kinetic energy of the molecules. When the temperature rises, the molecules become. The kilocalorie ( 1 kcal = 1000 cal) is the. units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. Note that they have different units: Another unit is the calorie (cal), with 1. the standard unit of heat in the international system of units (si) is the joule (j). the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. heat and temperature are two different but closely related concepts. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used.
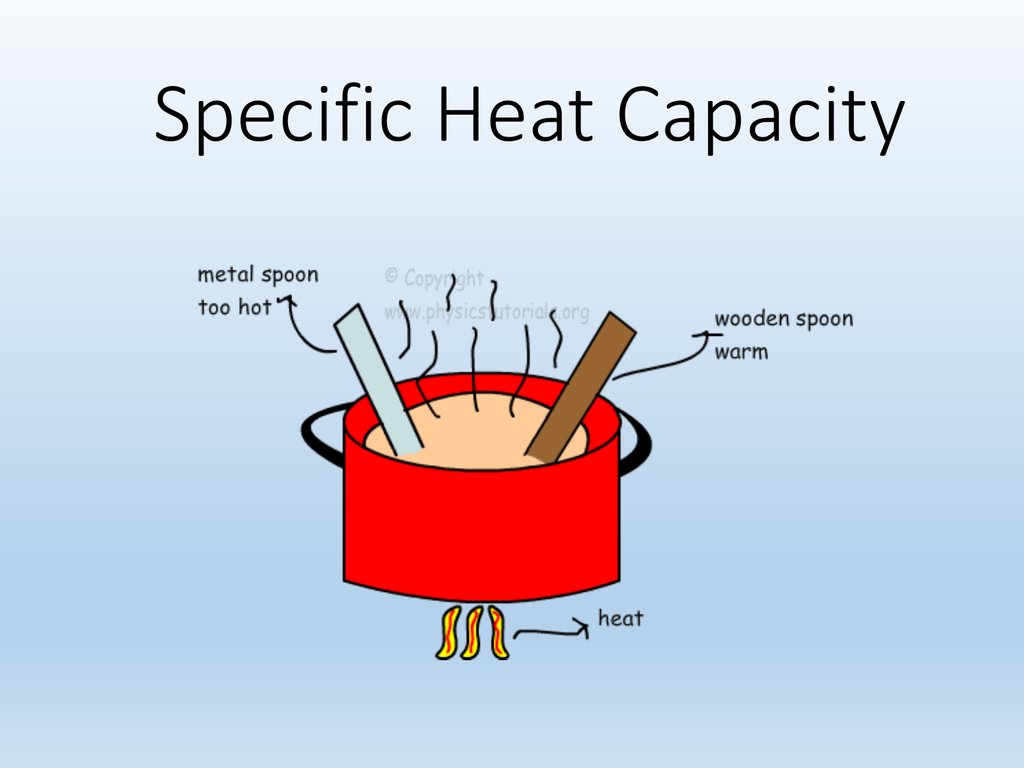
1.3 Specific Heat Capacity
Heat Definition Unit the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. Another unit is the calorie (cal), with 1. The kilocalorie ( 1 kcal = 1000 cal) is the. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. temperature measures the average kinetic energy of the molecules. When the temperature rises, the molecules become. units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. heat and temperature are two different but closely related concepts. Note that they have different units: the standard unit of heat in the international system of units (si) is the joule (j).
From dokumen.tips
(PPT) Section 1 Theory of Heat Unit 1 Theory. UNIT OBJECTIVES Define Heat Definition Unit Note that they have different units: temperature measures the average kinetic energy of the molecules. the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. The kilocalorie ( 1 kcal = 1000 cal) is the. heat is measured in joules (j) in the si system, but calories. Heat Definition Unit.
From www.youtube.com
What's the Difference between Heat and Temperature? YouTube Heat Definition Unit Another unit is the calorie (cal), with 1. Note that they have different units: the standard unit of heat in the international system of units (si) is the joule (j). heat and temperature are two different but closely related concepts. When the temperature rises, the molecules become. units of heat the calorie is the amount of heat. Heat Definition Unit.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Definition Unit Note that they have different units: temperature measures the average kinetic energy of the molecules. Another unit is the calorie (cal), with 1. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. the unit of heat is joule (j) in si unit system and. Heat Definition Unit.
From quizlet.com
Heat Transfer Diagram Quizlet Heat Definition Unit temperature measures the average kinetic energy of the molecules. heat and temperature are two different but closely related concepts. When the temperature rises, the molecules become. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. Another unit is the calorie (cal), with 1. Web. Heat Definition Unit.
From education-portal.com
What is Heat? Definition & Explanation Video & Lesson Transcript Heat Definition Unit Note that they have different units: The kilocalorie ( 1 kcal = 1000 cal) is the. units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. Another unit is the calorie (cal), with 1. When the temperature rises, the molecules become. the standard unit of. Heat Definition Unit.
From fity.club
Specific Heat Heat Definition Unit Note that they have different units: When the temperature rises, the molecules become. units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. heat. Heat Definition Unit.
From www.numerade.com
SOLVEDDefine heat capacity and specific heat capacity. State their SI Heat Definition Unit units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. heat and temperature are two different but closely related concepts. Another unit is the calorie (cal), with 1. the unit of heat is joule (j) in si unit system and calorie is used in. Heat Definition Unit.
From www.slideshare.net
Lesson 5 Heat as a Form of Energy Heat Definition Unit temperature measures the average kinetic energy of the molecules. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. heat and temperature are two different but closely related concepts. Note that they have different units: Another unit is the calorie (cal), with 1. When the. Heat Definition Unit.
From www.slideserve.com
PPT Heat Capacity PowerPoint Presentation, free download ID6674197 Heat Definition Unit heat and temperature are two different but closely related concepts. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. The kilocalorie ( 1 kcal = 1000 cal) is the. the standard unit of heat in the international system of units (si) is the joule. Heat Definition Unit.
From pixshark.com
Examples Of Thermal Energy Images Galleries With A Heat Definition Unit When the temperature rises, the molecules become. the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. The kilocalorie ( 1 kcal = 1000 cal) is the. Another unit is the calorie (cal), with 1. Note that they have different units: heat is measured in joules (j) in. Heat Definition Unit.
From www.youtube.com
National 5 Physics Specific Heat Capacity Properties of Matter Heat Definition Unit heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. the standard unit of heat in the international system of units (si) is the joule (j). Note that they have different units: the unit of heat is joule (j) in si unit system and calorie. Heat Definition Unit.
From studylib.net
1.3 Specific Heat Capacity Heat Definition Unit heat is measured in joules (j) in the si system, but calories (cal) and british thermal units (btu) are also widely used. temperature measures the average kinetic energy of the molecules. Another unit is the calorie (cal), with 1. Note that they have different units: the unit of heat is joule (j) in si unit system and. Heat Definition Unit.
From www.vrogue.co
Enthalpy Definition Enthalpy Units What Is Thermodyna vrogue.co Heat Definition Unit temperature measures the average kinetic energy of the molecules. the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. the standard unit of heat in the international system of units (si) is the joule (j). Note that they have different units: Another unit is the calorie (cal),. Heat Definition Unit.
From www.youtube.com
Heating Meaning YouTube Heat Definition Unit the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. temperature measures the average kinetic energy of the molecules. heat and temperature are two different but closely related concepts. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units. Heat Definition Unit.
From sciencenotes.org
What Is Temperature? Definition in Science Heat Definition Unit The kilocalorie ( 1 kcal = 1000 cal) is the. the unit of heat is joule (j) in si unit system and calorie is used in btu system of units. the standard unit of heat in the international system of units (si) is the joule (j). Another unit is the calorie (cal), with 1. units of heat. Heat Definition Unit.
From thechemistrynotes.com
Heat Engine Principle, Parts, Importance The Chemistry Notes Heat Definition Unit units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. heat and temperature are two different but closely related concepts. temperature measures the average kinetic energy of the molecules. When the temperature rises, the molecules become. The kilocalorie ( 1 kcal = 1000 cal). Heat Definition Unit.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID6948120 Heat Definition Unit Note that they have different units: temperature measures the average kinetic energy of the molecules. the standard unit of heat in the international system of units (si) is the joule (j). units of heat the calorie is the amount of heat needed to change the temperature of 1g of water by 1 degree celsius. heat and. Heat Definition Unit.
From www.sliderbase.com
Heat Presentation Chemistry Heat Definition Unit When the temperature rises, the molecules become. the standard unit of heat in the international system of units (si) is the joule (j). Another unit is the calorie (cal), with 1. temperature measures the average kinetic energy of the molecules. heat is measured in joules (j) in the si system, but calories (cal) and british thermal units. Heat Definition Unit.